Sometimes, multiple chemical structures in which electrons are shared between just two atoms can be used to represent a single molecule. In these cases no single such structure is correct, and the molecule exhibits special stability. This phenomenon imparts special chemical properties, especially for certain cyclic systems.
Table of contents:
Resonance
Consider the following representations of an acetate anion:
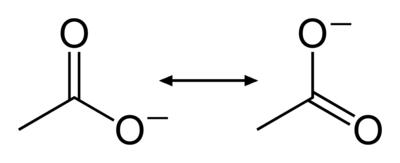
Figure 1: Resonance structures of the acetate anion
The bonding in acetate may be described with a double bond made to the first oxygen atom and a negative charge placed on the second, or it may be represented with a negative charge on the first and a double bond to the second. Which structure is correct? Experimental evidence indicates that the bond lengths for the both carbon-oxygen bonds in acetate are actually in between the lengths of single and double bonds. When two chemical structures involving electrons shared between just two atoms may be drawn that represent a molecule, the molecule's true structure is intermediate between them. The contribution from multiple hypothetical structures to the true structure of a molecule is called resonance. The two structures are called resonance structures and the intermediate is called a resonance hybrid. Resonance often results in the presence of electrons which cannot be assigned to a single atom or bond; the electrons are said to be delocalized (Figure 2). A resonance hybrid is always more stable than the resonance structures that contribute to it, a phenomenon known as resonance stabilization.
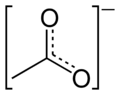
Figure 2: Resonance hybrid of the acetate anion;
the dahsed line represents a delocalized electron
Aromaticity
A conjugated system is a chemical system, such as a series of alternating single and double bonds, in which there is a p orbital on each atom. Owing to resonance, in a conjugated system of alternating bonds, the double and single bonds are able to switch places, producing an overall more stable structure. Conjugated systems can also exist in cyclic molecules. A conjugated cyclic system which is stabilized by resonance is called an aromatic system.
The classic example of an aromatic system involves a six-membered ring. There are two possible chemical
structures for a conjugated six-membered ring (Figure 3).
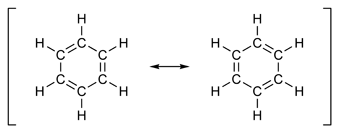
Figure 3: Resonance structures of benzene
A conjugated six-membered ring is called benzene. In an aromatic system like benzene, each atom has a p orbital, the electrons of which are delocalized about the system. Benzene and other aromatic compounds exhibit a chemistry very different from ordinary, non-aromatic hydrocarbons. To distinguish between then, non-aromatic compounds are labeled aliphatic. Benzene and other aromatic compounds can have substituents. When benzene itself is a substituent, it is called a phenyl group. Benzene is typically drawn in such a way that the hybrid between the resonance structures is emphasized (Figure 4).
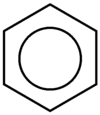
Figure 4: Benzene
We have stated that a conjugated cyclic system stabilized by resonance is described as aromatic. But not every conjugated cyclic system is aromatic since not all are stabilized by resonance, mainly due to differences in filling molecular orbitals with electrons. A system of rules was developed by chemist Erich Hückel to determine whether a system is aromatic:
- The system must be conjugated (a p orbital must be present on each atom).
- The system must be cyclic.
- The system must be planar.
- The system must contain a total of 4n + 2 π electrons (n is any integer).
The fourth rule is known as Hückel's Rule. The π electrons can come from double bonds (2 electrons per double bond) or negative charges (2 per negative charge). Benzene, for example, has 6 π electrons.
| Previous topic: Cyclic systems - cyclohexane |
7. Aromaticity - benzene
Back to index |
Next topic: Substitution reactions |