The alkanes we have studied so far have been of two types: linear and branched. There is a third type of alkane in which the molecule does not have ends but instead forms a ring. These molecules are called cycloalkanes (Figure 1).
Figure 1: Skeletal structure of cyclohexane, a cycloalkane
Stable cycloalkanes cannot be formed with carbon chains of just any length. Recall that in alkanes, carbon adopts the sp3 tetrahedral geometry in which the angles between bonds are 109.5°. For some cylcoalkanes to form, the angle between bonds must deviate from this ideal angle, an effect known as angle strain. Additionally, some hydrogen atoms may come into cloeser proximity with each other than is desirable (become eclipsed), an effect called torsional strain. These destabilizing effects, angle strain and torsional strain are known together as ring strain. The smaller cycloalkanes, cyclopropane and cyclobutane, have particularly high ring strains because their bond angles deviate substantially from 109.5° and their hydrogens eclipse each other. Cyclopentane is a more stable molecule with a small amount of ring strain, while cyclohexane is able to adopt th perfect geometry of a cycloalkane in which all angles are the ideal 109.5° and no hydrogens are eclipsed; it has no ring strain at all. Cycloalkanes larger than cyclohexane have ring strain and are not commonly encountered in organic chemistry.
Table of contents:
Cyclohexane
Most of the tiem, cyclohexane adopts the fully staggered, ideal angle chair conformation (Figure 2). In the chair conformation, if any carbon-carbon bond were examined, it would be found to exist with its substituents in the staggered conformation and all bonds would be found to possess an angle of 109.5°.
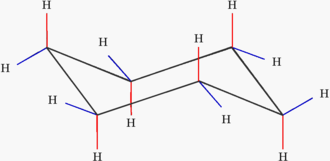
Figure 2: Cyclohexane in the chair conformation
In the chair conformation, hydogen atoms are labeled according to their location. Those hydrogens which exist above or below the plane of the molecule (shown with red bonds above) are called axial. Those hydrogens which exist in the plane of the molecule (shown with blue bonds above) are called equatorial.
Although the chair conformation is the most stable conformation that cyclohexane can adopt, there is enough thermal energy for it to also pass through less favorable conformations before returning to a different chair conformation. When it does so, the axial and equatorial substituents change places. The passage of cyclohexane from one chair conformation to another, during which the axial substituents switch places with the equatorial substituents, is called a ring flip.
Methylcyclohexane
Methylcyclohexane is cyclohexane in which one hydrogen atom is replaced with a methyl group substituent. Methylcyclohexane can adopt two basic chair conformations: one in which the methyl group is axial, and one in which
it is equatorial. Methylcyclohexane strongly prefers the equatorial conformation. In the axial conformation,
the methly group comes in close proximity to the axial hydrogens, an energetically unfavorable effect
known as a 1,3-diaxial interaction (Figure 3). Thus,
the equatorial conformation is preferred for the methyl group. In most cases, if the cyclohexane ring contains a subsituent, the substituent will prefer the equatorial conformation.
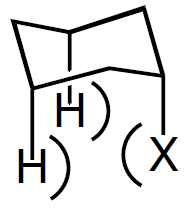
Figure 3: 1,3-diaxial interaction
| Previous topic: Conformational analysis of alkanes |
5. Cyclic systems - cyclohexane
Back to index |
Next topic: Aromaticity and benzene |